
The cookie is a session cookies and is deleted when all the browser windows are closed. The cookie is used to store and identify a users' unique session ID for the purpose of managing user session on the website. This cookie is native to PHP applications. These cookies do not store any personal information. This category only includes cookies that ensures basic functionalities and security features of the website. Necessary cookies are absolutely essential for the website to function properly. San Francisco, CA: Portola Pharmaceuticals, Inc May 2018. Andexanet Alfa for Acute Major Bleeding Associated with Factor Xa Inhibitors. Continued approval for this indication may be contingent upon the results of studies to demonstrate an improvement in hemostasis in patients.Ĭonnolly SJ, Milling, Jr, TJ., Eikelboom, et al.
To reduce thromboembolic risk, resume anticoagulant therapy as soon as medically appropriate following treatment with AndexXa ®.įDA gave this drug an accelerated approval and is the only approved product for reversal of apixaban and rivaroxaban. Also, monitor for symptoms and signs that precede cardiac arrest and provide treatment as needed.

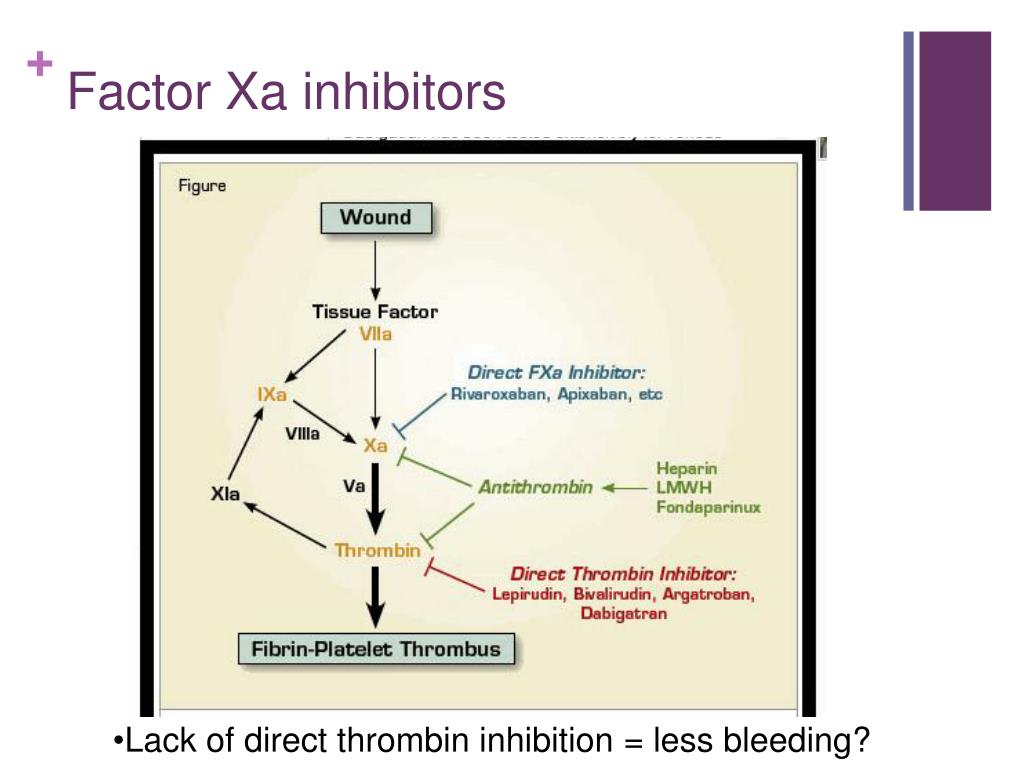
The package insert recommends to monitor for thromboembolic events and initiate anticoagulation when medically appropriate. Ischemic events, including myocardial infarction and ischemic stroke.Arterial and venous thromboembolic events.The side effect profile is low, however andexanet does carry the following warning that the drug is associated with serious and life-threatening adverse events, including: The package insert for AndexXa ® states an improvement in hemostasis has not been established. The completed trial in healthy volunteers and ongoing research trial with patients with acute major bleeds show overall efficacy in the agent’s ability to decrease anti-FXa activity. In addition, it inhibits the activity of Tissue Factor Pathway Inhibitor (TFPI), increasing tissue factor-initiated thrombin generation. It works as a reversal agent by binding and sequestering Factor Xa inhibitors. Andexanet alfa is a recombinant modified human Factor Xa protein that is catalytically inactive. Aandexanet alfa is indicated for patients who have been treated with rivaroxaban (Xarelto ®) or apixaban (Eliquis ®), and are in need of anticoagulation reversal due to life-threatening or uncontrolled bleeding. The first Factor Xa inhibitor reversal agent, andexanet alfa (brand name of AndexXa ®) received FDA approval in early May 2018.


 0 kommentar(er)
0 kommentar(er)
